The Download from ARVO 2023: AI and Interoperability Drive Breakthroughs in Ophthalmic Research
The Download from ARVO 2023: AI and Interoperability Drive Breakthroughs in Ophthalmic Research
The American Association for Research in Vision and Ophthalmology (ARVO) recently held its annual conference, showcasing the latest developments in the industry. Among the key takeaways were the crucial roles of artificial intelligence (AI) and interoperability in driving breakthroughs for ophthalmic research. In this blog, we will explore the importance of interoperability, the power of AI-based analysis, and the promise of new solutions on the horizon.
The Importance of Interoperability
Interoperability, the ability of different systems to exchange and interpret data seamlessly, has become a vital aspect of ophthalmic clinical research. It provides an essential environment for accurate, verifiable, and reproducible results. By boosting efficiencies across all aspects of clinical research and trials, interoperable systems empower research, especially by making more data available, accelerating workflows, shortening the length of clinical trials, lowering the associated costs, and ultimately leading to better patient outcomes.
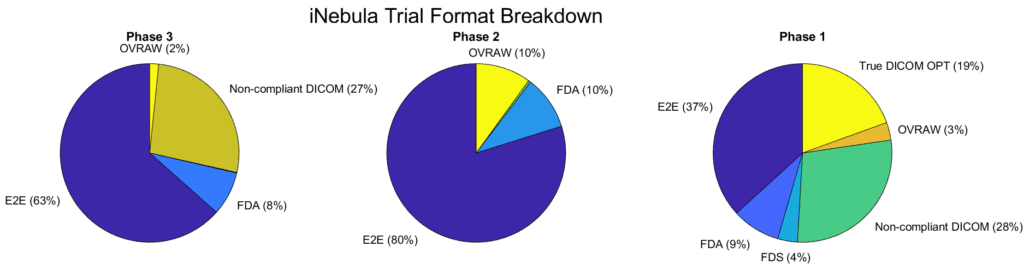
Yet despite the importance of interoperability, several critical challenges continue to hinder progress in clinical research and trials. This was touched upon at ARVO during the NEI’s director’s session, where the initiatives in this area were discussed. And this was also the theme of the ARVO follow-up SIG meeting on big data and interoperability that again focused on the impediment to clinical research and clinical trials in this area. In that meeting, Verana Health explained how they converted the IRIS registry “with help from each manufacturer”. This need for DICOM conversion directly impedes any workflow, as shown below based on three concluded trials, at different phases. Notice how much of the data is converted to true DICOM ahead of processing in Orion (Figure 1). Spoiler alert– it’s almost none. And why would you? It’s hard enough to get sites to follow established workflows for trial data export that invariably differ from trial to trial.
Figure 1–- Data processed through Orion, our clinical trial reading software, for three concluded clinical trials showing the image format breakdowns.
To summarize the critical interoperability obstacles voiced at ARVO:
- Failure to adopt established data standards: As the above figure shows, ophthalmic device manufacturers have been slow to adopt DICOM as a standard, whereas in radiology it is the default. This, as mentioned, is the key factor that hamstrings clinical research and trials.
- Data inaccessibility: Research data often remains siloed within individual institutions or organizations, resulting in limited access. This lack of transparency inhibits the ability to validate findings across multiple trials and creates laborious processes for data sharing and storage.
Promising Solutions on the Horizon
While interoperability challenges persist, promising solutions and trends are emerging:
- DICOM & HL7 Standards: The adoption of standardized data formats, such as DICOM and HL7, enhances interoperability by facilitating seamless integration and exchange of ophthalmic imaging and clinical data. Adoption of these data standards for all device manufacturers is a frequent topic of discussion in workshops, connect-athons, and conferences, such as ARVO.
- Electronic health record evolution and full platform visibility: The evolution of electronic health records (EHRs) offers opportunities for improved interoperability. By creating comprehensive platforms that provide full visibility across patient data, healthcare providers and researchers can access a holistic view of a patient’s medical history, aiding in research and treatment decisions.
- Distribution of data to a broader research audience: Efforts to establish data repositories and open-access platforms can facilitate broader data distribution. Through data-sharing, researchers accelerate discoveries by leveraging larger, more diverse datasets. And this, of course, is also requisite to the development of AI-based algorithms.
Interoperability Spotlight: Accelerating Rare Disease Research
Interoperability holds immense potential in accelerating rare disease research within the field of ophthalmology. Collaboration and data sharing are especially crucial in rare disease research, where individual datasets may lack statistical significance on their own. By bringing together disparate data sources, researchers can gain deeper insights into the causes, progression, and potential treatments for these conditions.
Aggregating and connecting data through clinical research networks can provide a much-needed platform for collaboration, knowledge sharing, and accelerating research and innovation. And given the low likelihood of each set of clinical research data sharing the same format, interoperable platforms bridge the gap while also offering centralized data storage, real-time data access, as well as accessibility from any device.
The Power & Limitations of AI-based Analyses
A discussion of interoperability would not be complete without including the role of AI. Most analysis tasks presented at ARVO use AI, as one would expect given the advances in image interpretation offered by deep learning-based techniques. But amid all the excitement, much of which is justified, it is worth recalling the so-called AI-chasm discussed by Pearse Keane and Eric Topol; namely, the bridge from exciting results to clinical deployment. A key contributor to this chasm, and one specific to ophthalmic research, is the difficulty in translating results from exciting studies on a limited validation set, to real-world deployment, confounding some of the best-resourced organizations in this space. Progress is also gated by regulatory bodies, and the emerging pattern in ophthalmology is that the first AI-based release defaults to the more stringent de novo approval pathway if there is no clear predicate.
At ARVO, you could pretty much place the application of AI into one of two categories: semantic segmentation; and disease diagnostics/prognostics. These were mostly applied to imaging data, but there is increasing interest in incorporating any available data into the inference process. For relevant data, the term oculomics has been coined, a play on genomics, referring to imaging and demographic information. In clinical trials, it is semantic segmentation that is of interest in an effort to generate new biomarkers; i.e., structural end points of relevance to disease progression. For these more advanced metrics, there is currently limited association with visual acuity outcomes, so it will be challenging to see intra-retinal fluid, for example, as a primary endpoint in the very near future. That said, the Appellis approval for dry AMD was groundbreaking in this sense as it did involve a structural parameter as a primary endpoint. This is an exciting blueprint for future studies showcasing how the science and regulatory bodies can move forward in concert. That is not to say that concerns about the discordance between the structural and functional outcomes do not exist.
In conclusion, ARVO 2023 was abuzz with industry innovators that are by integrating new techniques that harness the power of big data and AI-backed analyses to facilitate successful clinical trials and research. While there are the aforementioned bumps in the road, the pace of innovation is accelerating, which is good for our industry.
At Voxeleron, we strive to be among those crossing the chasm with clinically impactful methods to bridge the data standardization gap we see in ophthalmology and offer true AI-backed interoperability.
Watch this video to learn more about our iNebula clinical trial management or get in touch to schedule your demo today.
Sources:
https://www.aao.org/eyenet/article/interoperability-putting-pieces-together
https://www.aao.org/eyenet/article/mips-2022-whats-new-promoting-interoperability
https://jamanetwork.com/journals/jamaophthalmology/article-abstract/2793765