Addendum to the Enabling Technologies of OCT-A Post
Addendum to the Enabling Technologies of OCT-Angiography Post
One of the most exciting new applications of optical coherence tomography (OCT) imaging has been OCT angiography (OCT-A), the ability to non-invasively visualize blood flow [Lumbroso 2016]. As a relatively new technology, it is a hot topic of clinical research with multiple papers paving the way for clinical application [Fang 2016]. Its adoption in clinical trials is hamstrung somewhat by the inability to reliably quantify parameters derived from the OCT-A data, despite significant research effort [Jia 2015].
The potential clinical utility is no doubt exciting, and as described in this post, the underpinning technologies are critical toward reliable OCT-A image generation, quantification and, therefore, interpretation. As an addendum to that post, we review here a recent report that underlines just how critical one of these technologies – layer segmentation – is to the clinical interpretation of OCT-A data. To the best of our knowledge, this is the first comparison of more than one OCT-A instrument and offers tremendous insight into their relative performance while highlighting concerns regarding their automated algorithms.
Spaide and Curcio 2017
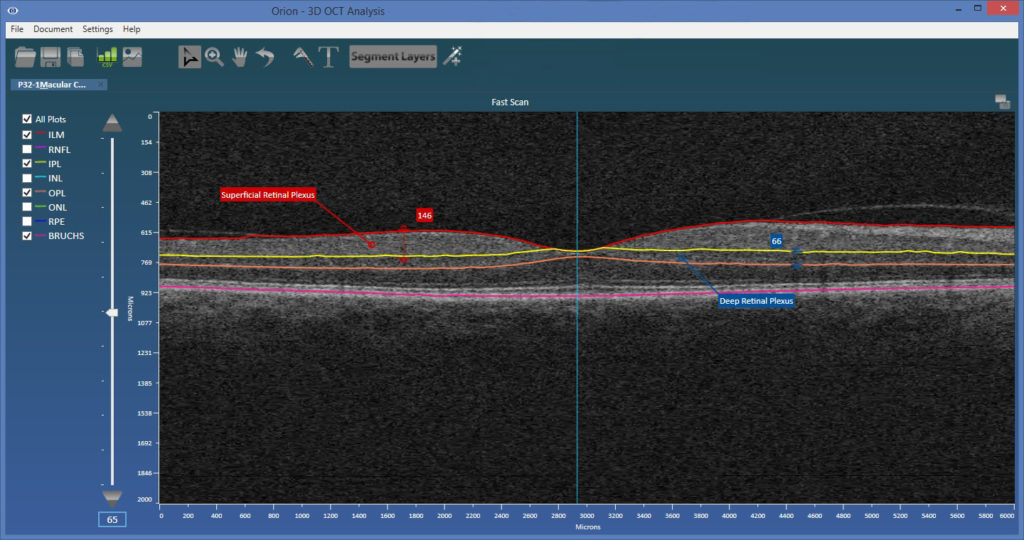
OCT-A looks primarily at blood flow within different retinal plexi. Spaide and Curcio looked at three OCT-A devices and compared their definitions of two important vascular plexi as delineated by automated layer segmentation. This is done in a healthy eye of a single patient, imaged using the Cirrus 5000 (Carl Zeiss Meditec), the RTVue XR Avanti (Optovue), and the Triton DRI OCT (Topcon Medical Systems).
The plexi considered were [Lumbroso 2016] (see also the figure below):
- The superficial retinal plexus – inner limiting membrane (ILM) to the outer boundary of the inner plexiform layer (IPL).
- And the deep retinal plexus – the outer boundary of the IPL to the outer boundary of the outer plexiform layer (OPL).
Unfortunately:
they all incorrectly segmented the central macula of normal eyes. As a consequence, imaging of the superficial and deep vascular plexus in the central macula and any abnormalities contained therein were not accurately characterized.”
Cirrus 5000
![In The B-Scan Image From The Zeiss Cirrus 5000 Platform, The Segmented Superficial Slab Appeared To Extend From The Ilm To The Bottom Of The Inner Nuclear Layer (Inl). The Slab For The Deep Plexus Was Shown To Extend From The Internal Aspect Of The Inl To Well Below The Opl. The Lower Boundary Of The Superficial Slab Measured 117 Μm Below The Ilm In The Central Fovea, Which Is Illustrative Of The Error In The Segmentation At The Foveal Center.” [Spaide 2017]](https://voxeleron.com/wp-content/uploads/2017/01/CZM-angio-300x202.jpg)
RTVue XR Avanti
![Optovue Device Appeared To Segment The Superficial Slab From The Ilm To The Inner One-Third Of The Inl. The Deep Slab Was Segmented From The Inner One-Third Of The Inl To Below The Opl. The Bottom Edge Of The Superficial Slab Was 55 Μm Below The Ilm In The Central Fovea. [Spaide 2017]](https://voxeleron.com/wp-content/uploads/2017/01/Optovue-angio.jpg)
Triton DRI OCT
![The Topcon Instrument Segmented The Superficial Slab From What Appeared To Be The Top Of The Iilm To Just Below The Ipl. The Deep Slab Was Segmented From Just Below The Ipl To Just Below The Opl. The Bottom Edge Of The Superficial Slab Was 40 Μm Below The Ilm In The Central Fovea. [Spaide 2017]](https://voxeleron.com/wp-content/uploads/2017/01/Topcon-angio.jpg)
Discussion
Clearly errors in the definition of each plexi will result in erroneous en face images, that cannot then be accurately quantified. Perhaps then this is just an outlier, and, in normal eyes at least, the results are more reliable? Not so it would seem:
Although 1 eye was used to show the defects in segmentation, the same is seen in other normal eyes.”
It is not clear then what actually happens in cases of pathology. Yet the user can always resort to manual correction of the layer boundaries. This, however, takes time and is subjective, but at least it facilitates the recovery of a corrected result. Well, perhaps it doesn’t after all:
… because the error is between the segmentation surfaces and the actual anatomy, the error varies by location. Therefore, manually adjusting the level of the segmentation lines is not a simple fix for the underlying error.”
That is, you can only actually move the boundaries up or down, and efficient, intelligent editing simply does not exist.
The concluding remarks are, as might be expected, pretty hard hitting:
Estimates of retinal vascular abnormalities, such as microaneurysms, or even measurement of normal features, like the size of the foveal avascular zone in the superficial vascular plexus and deep vascular plexus, will be incorrect because of the errors in segmentation.”
Such cautionary words from such expert practitioners should be heeded, especially in light of all the on-going OCT-A based clinical trials. More optimistically, clinicians are experts in making effective use of imperfect technologies and understanding the utility that lies within. Subsequently, OCT-A can only evolve and benefit from expert input and guidance as the next generation devices are developed. In the meantime, however, it would not be surprising if most practicing ophthalmologists are slow to warm to OCT-A as, given the performance in ideal conditions, the technology has well noted shortcomings that put the onus firmly on the clinician’s expert interpretation. That would actually be fine if the required expertise were solely in the clinical domain, but it currently goes beyond that, requiring a fairly sophisticated understanding of the algorithmic steps that created the image in order to recognize artifact as and when it presents.
References
[Lumbroso 2016] – Practical Handbook of OCT Angiography, B.Lumbroso, D. Huang, C.J. Chen, Y.Jia, M.Rispoli, A.Romano, N.K.Waheed, Jaypee Brothers Medical Publishers (P) Ltd, May 2016. [Hecht 2017] – Retinal layers thickness changes following epiretinal membrane surgery, Hecht I, Yeshurun I, Bartov E, Bar A, Burgansky-Eliash Z, Achiron A. Eye (Lond). 2017 Nov 10. [Fang 2016] – Clinical applications of OCT angiography, Fang PP et al., Ophthalmologe. 2016 Jan;113(1):14-22. [Jia 2015] – Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Jia Y et al., Proc Natl Acad Sci U S A. 2015 May 5;112(18). [Spaide 2017] – Evaluation of Segmentation of the Superficial and Deep Vascular Layers of the Retina By Optical Coherence Tomography Angiography Instruments in Normal Eyes.Richard F. Spaide and Christine A. Curcio. JAMA Ophthalmol. Published online January 12, 2017.
