Orion as Clinical Trial Tool for Cross-Platform Analysis
This past week clinical researchers from the Jacobs Retina Center at the Shiley Eye Institute of the University of California San Diego published a thorough evaluation of our Orion software to assess its suitability for research settings such as clinical trials. Published in Nature’s Scientific Reports (available at this link), a comparison is made to the device manufacturer’s software (Heidelberg Engineering, Germany) in normal and diseased eyes. This seminal paper addresses the paucity of publications looking at retinal segmentation in real-world pathology cases with particular emphasis on intra-retinal layer performance. In doing so the study concludes that several advantages are offered using Orion, our device independent analysis tool, the primary one being overall accuracy.
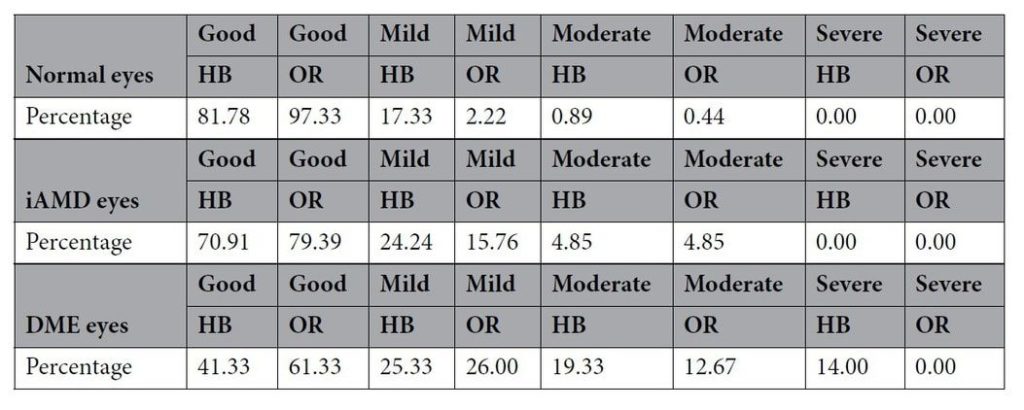
In total, 43 normal, 33 intermediate AMD (iAMD) and 39 eyes with Diabetic Macular Edema (DME) were assessed independently by two masked manual graders, with a senior grader adjudicating any discrepancies in the findings. Table 1 summarizes the qualitative evaluation that found, in all categories, the Orion software (OR) had lower error rates; and importantly, no severe errors in any category.
A quantitative analysis told the same story with Orion being significantly better than the proprietary software in the segmentation of NFL and INL layers in normal eyes and significantly better for NFL in iAMD and for INL and OPL layers in DME eyes. While all other layers had lower errors rates, in all categories, there was no significance to the differences.
Orion has been involved in many studies and generated a lot of literature, this paper is the first to compare intra-retinal segmentation in diseased retina to manual segmentations and the device’s software. It concludes that:
the cross-platform [Orion] software was subjectively better at segmenting retinal layers than the proprietary software in both normal and particularly in diseased eyes.
And that:
the cross-platform [Orion] software offers a reliable alternative for intra-retinal segmentation which may be particularly useful for research studies and clinical trials as its clinical use is not yet approved.
There are many other work-flow advantages offered by Orion, over and above freeing the user from slow, inaccurate, single-vendor software solutions – we touch upon these advantages in this article. It is clearly beneficial to use software that performs best, but also one where the same landmarks are segmented regardless of the OCT device used and, when editing is needed, tools like intelligent editing can be applied quickly to get to the correct endpoint, as is vouched for here. Time is money in clinical trials and a tool like Orion can significantly speed up the time to get more accurate and more relevant endpoints. Those cost savings can have a significant impact on a study’s bottom line and can be realized today. Soon, however, we will also be offering this functionality, as well as AI-based endpoints and inference engines for both AMD and glaucoma, hosted securely in the in the cloud!
